

Original file (SVG file, nominally 216 × 124 pixels, file size: 10 KB) File information. The sulfenic acids, in turn, spontaneously rearrange to form syn-propanethial-S-oxide, the chemical that triggers the tears. But this would at least separate it from the solid components of the onion and any non-volatile liquid components, so it would be a good start. Size of this PNG preview of this SVG file: 216 × 124 pixels. syn-Propanethial S-oxide (C 3 H 6 OS), a member of a class of organosulfur compounds known as thiocarbonyl S-oxides (formerly 'sulfines'), is a gas that acts as a lachrymatory agent (triggers tearing and stinging on contact with the eyes).The chemical is released from onions, Allium cepa, as they are sliced. It wouldn't be perfectly pure, since you would condense some water vapour too, along with any other volatile compounds evaporating from the onions. However, the boiling point is estimated to be not far above 100 degrees C, so you would at least condense it as a liquid. I can't find any data on what the freezing point might be, so I don't know if the dry ice would freeze it.
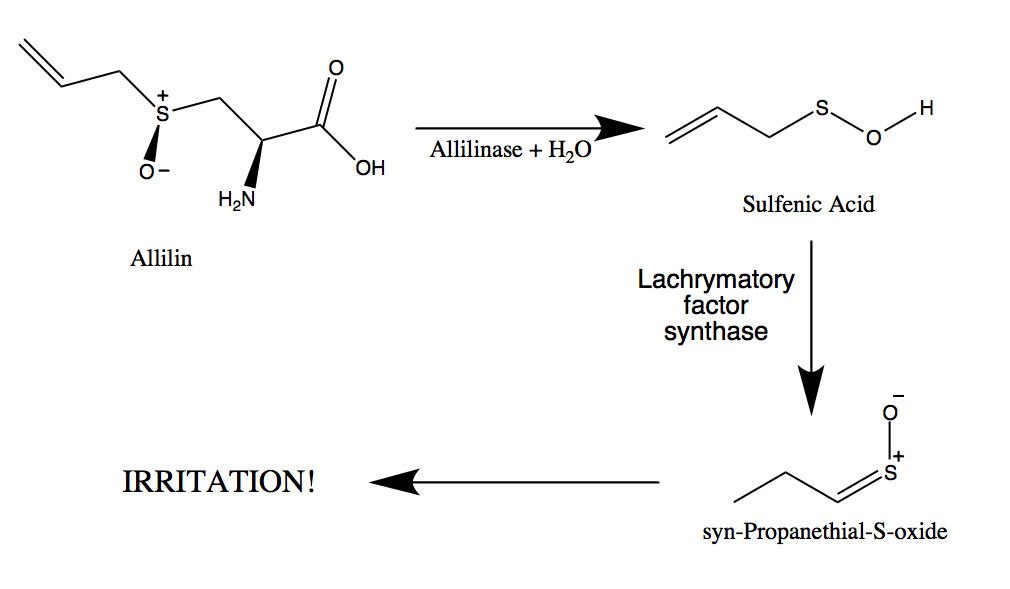
That way, the upper bowl would act like a big "cold finger", condensing the syn-propanethial-S-oxide vapours onto the underside of it. syn-Propanethial S-oxide, a member of a class of organosulfur compounds known as thiocarbonyl S-oxides, is a volatile liquid that acts as a lachrymatory. Then put a layer of dry ice into another identical bowl, and rest this on top of the first one, with a small air gap between the layer of onions and the bottom surface of the upper bowl. So one possible way would be to spread out the freshly grated onions in a large, wide glass or stainless steel bowl. Are you a university or school looking for an online tutoring partnership Talk to us Home What Neutralizes The Syn Propanethial S Oxide Chemical Qa. Sulfenic acids are unstable and spontaneously rearrange into syn-propanethial-S-oxide. As onions are sliced, cells are broken, allowing enzymes called alliinases to break down amino acid sulfoxides and generate sulfenic acids. Then you would have to capture the vapour coming off. Syn-propanethial-S-oxide is a volatile gas that triggers the tears when an onion is cut. You would have to freshly slice some onions into really small pieces for efficient extraction, so maybe a fine grater would be the best idea rather than chopping with a knife.

Technically you can extract it from onions, but it would require some fairly elaborate equipment since the compound is only released when onion cells are cut into.


 0 kommentar(er)
0 kommentar(er)
